
Thus, the pH of a solution that is 0. The p-function of a number X is written as p X and is defined as When working with concentrations that span many orders of magnitude, it is often more convenient to express the concentration as a p-function. CASC built in database of over 300 compounds with density tables makes all calculations fast - and accurate.

Graph of versus volume of NaOH and pH versus volume of NaOH for the reaction of 0.10 mol/L HCl with 0.10 mol/L NaOH. Calculate exact pH of any mixture of multiprotic acids and bases. For the last ten additions of NaOH, however, changes in the are too small to be seen.įigure 1. NOTE: Our new and improved water calculator more accurately predicts mash pH. This calculator is good for basic water adjustments and targeting a flavor profile only. Calculates balance of flavor ions and checks for harmful levels. We can easily follow changes in the over the first 14 additions of NaOH. Water chemistry calculator for beer brewers targeting certain mineral levels. The solute is assumed to be either strong acid or strong base. The initial is 0.10 mol/L, and its concentration after adding 75 mL of NaOH is 5.0 × 10 -13 mol/L. Study Chemistry pH of a strong acid/base solution This online calculator calculates pH of the solution given solute formula and solution molarity. Such is the case in Figure 1, where the molar concentration of H + is plotted ( y-axis on left side of figure) as a function of the volume of NaOH added to a solution of HCl. If, entered concentrations are not matched to a buffer solution. pH pKa CH3COOH + log 10 ( CH 3 COO - / CH 3 COOH) pH 4.75 + log 10 (0.02/0.05) pH 4.75 + log 10 (0.4) pH 4.750 + (-0.398) pH 4.352 Example 2: Preparing Buffer solution with ammonia and hydrochloric acid You were given 40 cm 3 of 0.1 M ammonia solution and you have added 10 cm 3 of 0.1 M HCl solution. The pH can be calculated using: pH -log 10 H + where H + concentration of H + ions (mol dm -3) The pH can also be used to calculate the concentration of H + ions in solution by rearranging the equation to: H + 10 -pH Worked Example: Calculating the pH of acids Answer pH -log H + -log 1.32 x 10 -3 2. pKa value will be filled automatically, when you select the acid / base combination. Fill concentrations of each acid and base.

Select the acid / base combination of buffer solution. If we are interested in viewing the progress of the reaction graphically, we might wish to plot the reactant’s concentration as a function of time or as a function of the volume of a reagent being added to the reaction. Please follow following steps to determine pH of buffer solution.
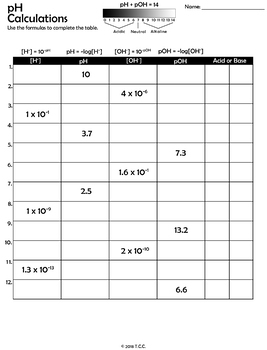
For example, a reactant’s concentration may change by many orders of magnitude. Sometimes it is inconvenient to use the concentration units.


 0 kommentar(er)
0 kommentar(er)
